FSS 50DM™: Direct Food Contact Certification
Determine compliance of the coating to FDA extraction tests for Condition B use with Type II food

SAMPLE DESCRIPTION:
The Client submitted twelve 1/2 pint jars coated with VersaFlex FSS 50DM™, Project 13-0089.
REQUEST:
Determine compliance of the coating to FDA extraction tests for Condition B use with type II food.
METHOD:
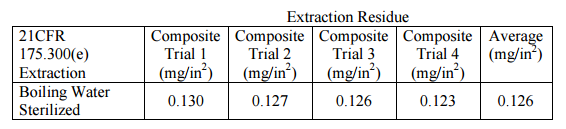
The samples were subjected to the extraction test outlined in 21CFR 175.300(d) Table 2, Condition B and described in 21CFR 175.300(e)(4)(ii) to simulate boiling water sterilization. A measured volume of boiling distilled water was added to each of twelve test vessels. Each vessel was covered with foil, and placed on a rack in a pressure cooker in which a small amount of distilled water was boiling. The pressure cooker was closed without sealing, and was allowed to continue to heat at boiling for 30 minutes. Subsequently, the liquid from every three vessels was composited, and then evaporated to dryness, giving quadruplicate trials. Mass of residue per square inch of exposure surface was calculated from the experimental data.
RESULTS:
Sample: VersaFlex FSS 50DM™, Project 13-0089
Requirements per 21CFR 175.300(c) (maximum allowed for any extraction condition):
Single-use containers ≤ 1 gallon: 0.5 mg/in² AND*
Single-use containers > 1 gallon: 1.8 mg/in² AND*
Repeat-use: 18 mg/in² AND*
* ALSO 0.005% of water capacity of container, in milligrams, divided by the area of the contact in square inches.
CONCLUSION:
The submitted coating, “VersaFlex FSS 50DM”, meets the extraction requirements of 21CFR 175.300(c) for use as a coating in contact with Type II foods under Condition B (boiling water sterilized) for single and repeated use container applications. If the “VersaFlex FSS 50DM” coating chemistry is among the chemistries covered by 21CFR 175.300, then the coating complies with 21CFR 175.300 for Type II food types stored under Condition B in contact with the coating.